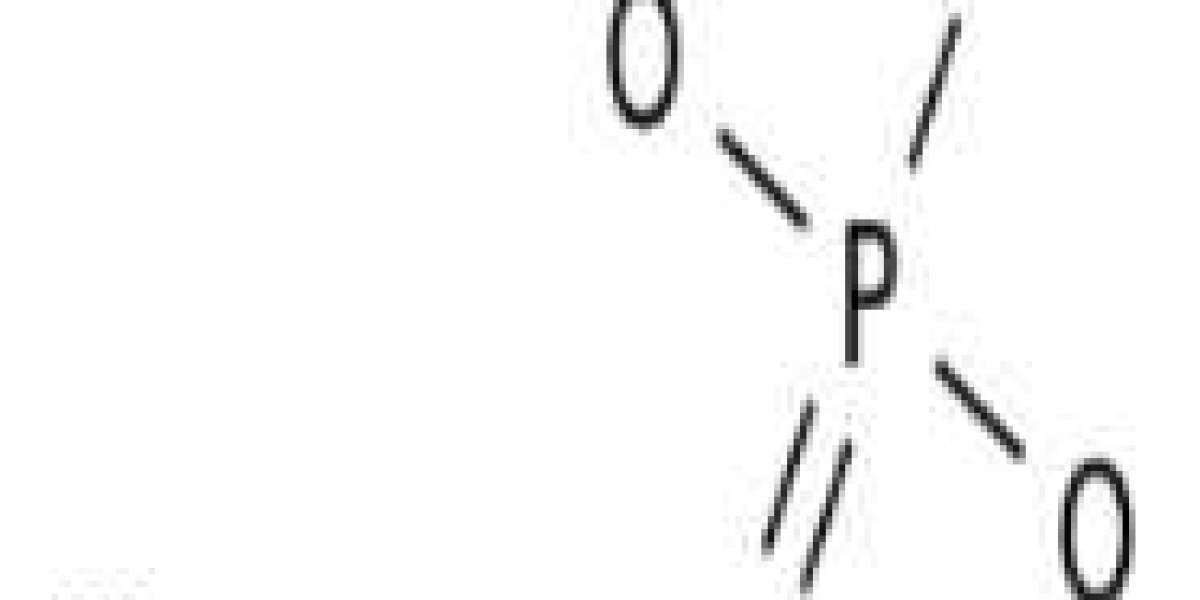
Magnesium Phosphate - mg3 po4 2
What is Magnesium Phosphate?
Magnesium Phosphate, mg3 po4 2 is an important mineral found in bones, in the seeds of many plants, and in a number of minerals. It is taken as a source of supplemental dietary magnesium. Tribasic magnesium phosphate may be prepared by the interaction of magnesium chloride or sulphate with the phosphate salt. The solution is easily prepared by agitating some salt with carbon dioxide-free water for several hours.
Chemical Properties of Magnesium Phosphate – mg3 po4 2
Magnesium Phosphate is a salt when treated with water forms phosphoric acid and magnesium hydroxide. The chemical equation is given below.
Mg3(PO4)2 + 6H2O → 2H3PO4 + 3Mg(OH)2
Magnesium Phosphate reacts with hydrochloric acid forms phosphoric acid and magnesium chloride.
Mg3(PO4)2 + 6HCl → 3MgCl2 + 2H3PO4
Uses of Magnesium Phosphate – mg3 po4 2
Used as a source for both magnesium and phosphorus. It is used in the minimal supplements.
Used in the prevention of vitamin E deficiency.
Used in foods at levels not to exceed current good manufacturing practice. The ingredient also may be used in infant formula.
Used as a nutrient, pH control agent and stabilizer.
Is mg3 po4 2 soluble or insoluble in water? Perhaps this is the question that you want to know. So, based on the principles of solubility and the elaborated solubility chart, magnesium phosphate or mg3 po4 2 is insoluble in water. However, a small amount of mg3 po4 2 might dissolve but it is not counted. Let's discuss more on whether mg3 po4 2 is soluble in water or not.
What makes mg3 po4 2 soluble or insoluble in water?
As said, magnesium phosphate is insoluble in water. But it is important to understand what makes mg3 po4 2 soluble or insoluble in water. While magnesium phosphate is considered an ionic compound, the ions present in the compound don't dissociate in water. Some basic principles of solubility have to be taken into consideration.
To begin with, magnesium phosphate is a kind of crystalline white powder. Surprisingly, a majority of the phosphate salts are soluble in water. Whereas mg3 po4 2 is insoluble in water. This is because a majority of the Mg salts won't be soluble in water. Besides, Mg cations don't dissolve in water in most situations.
So, now you know if mg3 po4 2 is soluble in water or not. But why is it so? Is there some specific explanation for this? To get the right explanation, you can research the solubility rules for different compounds, especially the ionic ones. Hence, according to the rules, phosphate salts and carbonate salts are insoluble in water.
mg3 po4 2 soluble or insoluble in water: Understanding the properties
mg3 po4 2 is a white crystalline powder that has a melting point of about 1457K. Most of the PO salts are soluble in water. But mg3 po4 2is not soluble in water because Mg salts are insoluble in water. Additionally, the Mg cations are not soluble in water most of the time. Rather, it gets dissolved in certain salt solutions.
The molar mass of magnesium phosphate is 262.86g/mol. mg3 po4 2doesn't have a constant or specific boiling point. That's because it dissipates before attaining a definite boiling point. However, Mg3(PO4)2 melts at 1188 degrees Celsius. Both boiling and melting points of mg3 po4 2 depend on several factors such as pressure, heating rate, concentration, crystal structure, and impurities.
Although it is good to know whether mg3 po4 2 is soluble in water or not, let's know where the compound is utilized most.
Mg3(PO4)2 and its uses
While you got your answer if mg3 po4 2 is soluble in water or not, it is good to know about the uses of magnesium phosphate.