What is the "isobutylene" I often hear in the news?
It is one of the largest industrial chemicals, despite the fact that under appropriate conditions, it can easily cause explosions... such as the recent fire at a Texas chemical plant.
A chemical plant in Texas has exploded and caught fire, using the highly flammable gas isobutylene as fuel, resulting in the death of one employee. The public is puzzled about why such a dangerous flammable gas is produced in such large quantities.
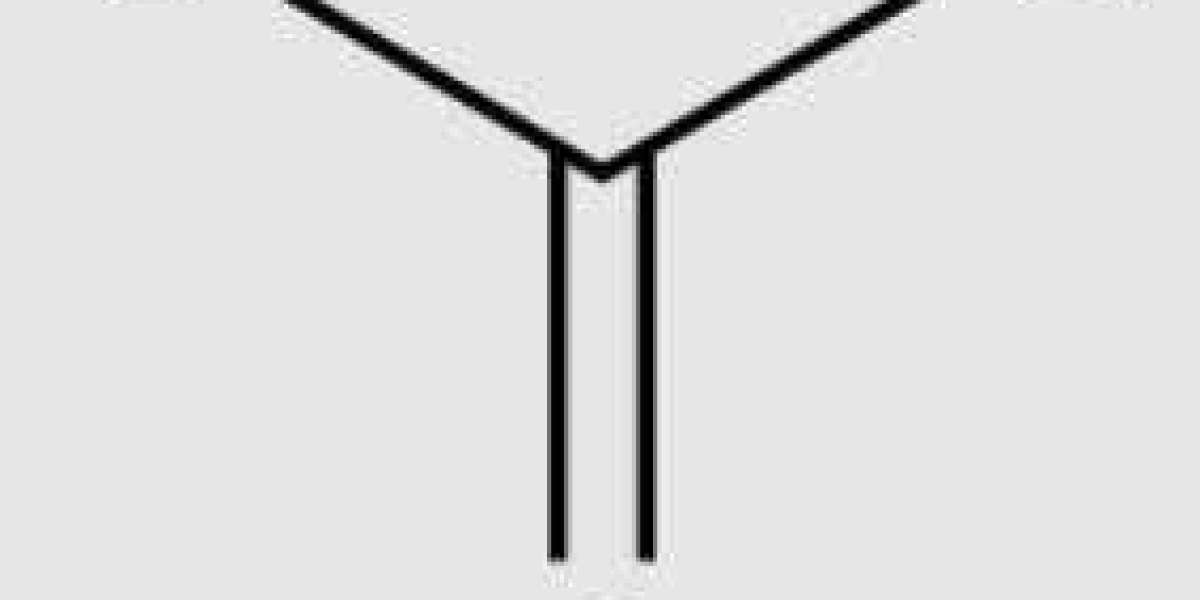
Isobutene (also known as 2-methylpropene, isobutylene, and γ- Butene, because chemists are not good at adhering to a naming system, is a colorless gaseous hydrocarbon at room temperature.
Its flash point is -80 ℃, which means that above this temperature, if there is an ignition source present, isobutylene will catch fire. Due to the coldest recorded temperature on Earth being -89 ˚ C. Isobutene is almost always only one spark away from flames on Earth.
Isobutene is widely used in the synthesis of many things, largely because it contains a double bond that can easily react to form other products. For example, it can react with ethanol to produce ethyl tert butyl ether (ETBE), which is a gasoline additive that can increase the octane number and make the fuel more resistant to impact or spontaneous combustion. Alternatively, it can combine with itself to form long chains to form a polymer, butyl rubber.
Butyl rubber is very useful. It is sealed, so it can be used to make sealing rings, window seals or cling films, as well as bottle stoppers, kick balls, and tires. It is odorless and odorless, so it can be added to chewing gum. Once chewed, it can be collected and recycled to recover isobutylene.
Like other forms of rubber, butyl rubber also decomposes when exposed to solvents such as ammonia, but the decomposition rate is much slower than other rubbers. This makes it widely used in protective clothing and gas mask.
Where do these isobutenes come from? Natural gas. Butane (the fuel in Zippo lighters) can be extracted from extracted natural gas, converted to tert butanol, and then converted to isobutylene.
So, although it is not the most sustainable chemical, the multiple uses of isobutylene can explain its large-scale production. Unfortunately, due to its highly flammable nature, precautions need to be taken to avoid fires like those that occurred in Texas.