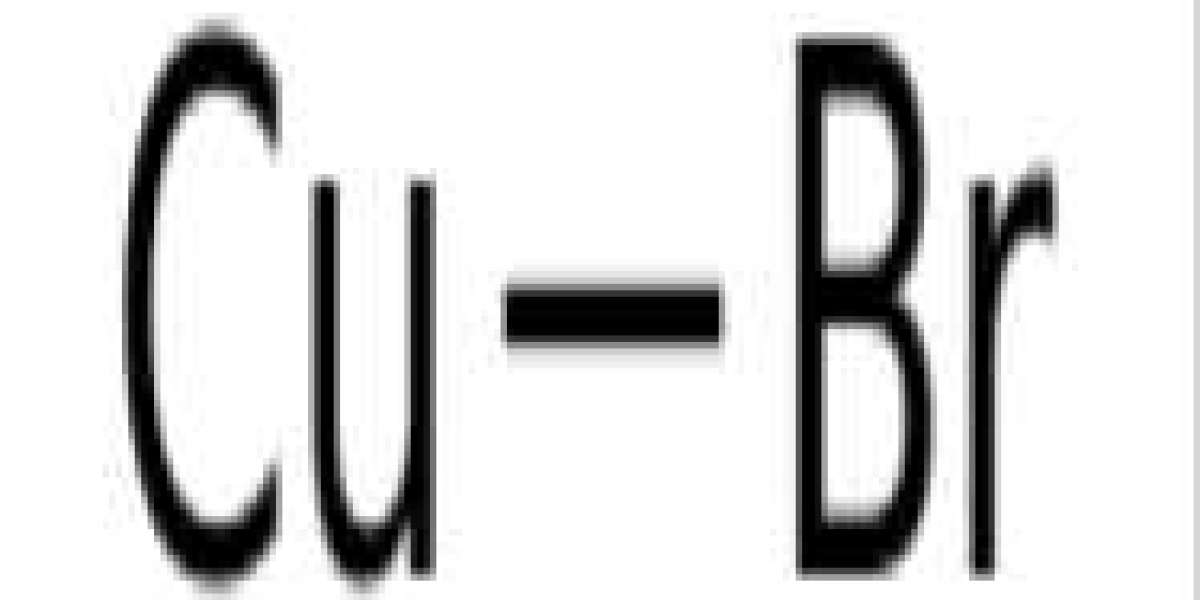Copper(I) bromide is a compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure similar to zinc sulfide. This compound is widely used in the synthesis of organic compounds and as a laser medium in copper bromide lasers.
The compound is white, but samples are often colored by the presence of copper(II) impurities. [3] Copper(I) ions are also readily oxidized in air. It is usually prepared by reducing copper salts with sulfites in the presence of bromide. [4] For example, reduction of copper(II) bromide with sulfite yields copper(I) bromide and hydrogen bromide:
2 CuBr2 + H2O + SO2−
3 → 2 CuBr + SO2−
4 + 2 hydrobromic acid
Copper bromide is insoluble in most solvents due to its polymeric structure, which has four-coordinated tetrahedral copper centers interconnected by bromide ligands (ZnS structure). After treatment with a Lewis base, CuBr was transformed into a molecular adduct. For example, a colorless complex is formed with dimethyl sulfide: [5]
CuBr + S(CH3)2 → CuBr(S(CH3)2)
In this coordination complex, copper is dicoordinated and has a linear geometry. Other soft ligands provide related complexes. For example, triphenylphosphine yields CuBr(P(C6H5)3), although this species has a more complex structure. Thermal excitation of copper(I) bromide vapor produces a blue-violet emission that is more saturated than the known emission of copper(I) chloride. [6] Copper(I) bromide is therefore a favorable emitter in pyrotechnic flames.
The effect of oxygen on the conductivity of CuBr at 420°C was investigated over a wide range of oxygen partial pressures (10−20PO210−3 atm). Although no effect on the overall conductivity (known to be mainly ionic conductivity) was observed, the effect of oxygen can be detected in the amperometric measurements of (-)Cu|CuBr|C(+) type cells because PO210 −17 ATMs. These observations are interpreted as the formation of acceptor O'Br due to the dissolution of oxygen on the Br sublattice, which compensates for the relatively low electron density n due to the formation of non-stoichiometric CuBr1−δ. The finding of n-type semiconductors is contrary to previous interpretations of CuBr polarization measurements. This discrepancy could be explained by decomposition reactions occurring at the graphite electrodes, which had been overlooked before. Furthermore, although a layer of Cu2O was observed to form at the Cu/CuBr interface, this is not the reason for the current dependence on PO2 in the above cells.