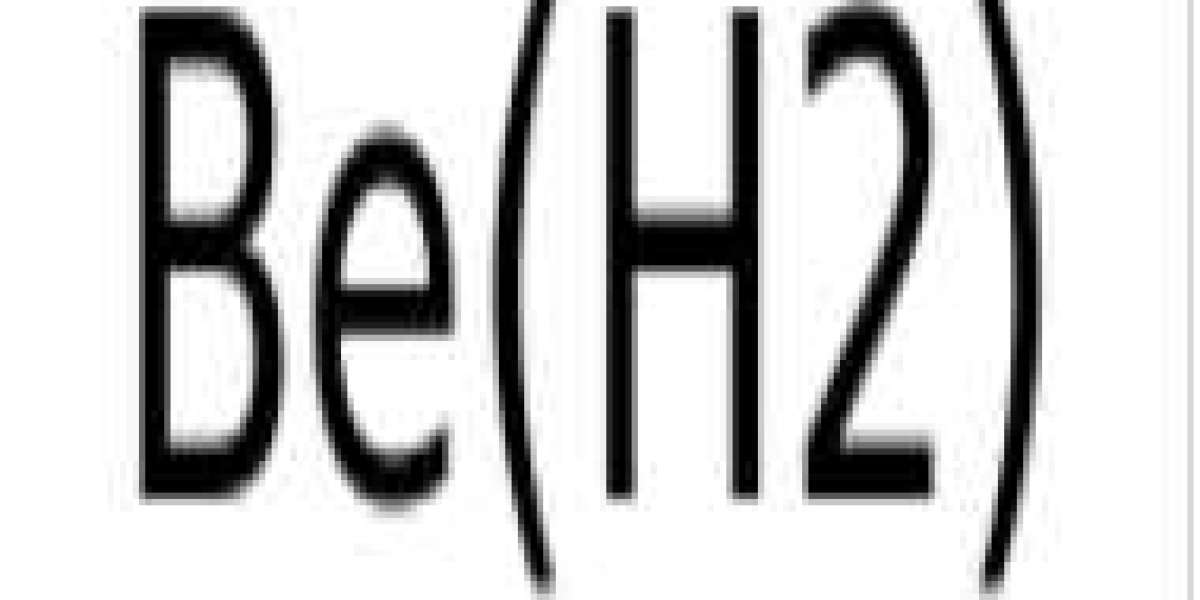Beryllium hydride is a hydride of beryllium. It is commonly used in rocket fuel. Beryllium is a light alkaline earth metal with atomic number 4. It is a relatively rare element found naturally only in combination with other elements in minerals.
BeH2 Lewis Structure, Molecular Geometry, Hybridization and Polarity
BeH2 is known as beryllium hydride or beryllium dihydride. It is an inorganic compound that belongs to the class of alkaline earth metal hydrides. It appears as an amorphous white solid at standard temperature and pressure. It also exists in the aggregated form of (BeH2) n.
Beryllium hydride is prepared by reacting dimethyl beryllium (Be(CH3)2) with lithium aluminum hydride (LiAlH4).
Be (CH3)2 + LiAlH4 —– BeH2 + LiAl (CH3)2H2
Beryllium hydride decomposes into beryllium hydroxide and hydrogen gas in water. Insoluble in toluene and ether. The molar mass of beryllium hydride is 11.03 g/mol. It decomposes at a melting point of 250 °C.
Beryllium hydride is a Lewis acid and thus forms dimeric or trimeric adducts with Lewis bases such as dimethylamine and trimethylamine, respectively.
Let us discuss the chemical bonds in the beryllium hydride molecule.
We will first draw its two-dimensional structure, the Lewis structure, according to the octet rule. We will then learn about its three-dimensional structure, the actual shape of the molecule, through VSEPR theory and VBT. Finally, we will learn whether the beryllium hydride molecule is polar or non-polar.
BeH2 Lewis structure
A Lewis structure is the simplest representation of any molecule and it includes the atoms of the molecule and the valence electrons of the atoms.
Why valence electrons and not core electrons?
Because only valence electrons (electrons that exist in the outermost shell of an atom) are available to form a chemical bond between two atoms. Core electrons are tightly bound to the nucleus and thus do not contribute to chemical bonds.
Not all valence electrons of an atom participate in bond formation. Some of them can remain unbonded and act as lone pairs.
In the Lewis structure of an atom, valence electrons are represented as points around the atom. Therefore, it is also called electron dot structure.
Let us draw the Lewis structure of the beryllium hydride molecule.
Beryllium atoms belong to group 2 (alkaline earth metals) of the modern periodic table of elements, and hydrogen atoms belong to group 1 (alkali metals) of the modern periodic table of elements.
Therefore, Be and H atoms have 2 and 1 valence electrons, respectively.
A beryllium hydride molecule consists of one beryllium atom and two hydrogen atoms. Therefore, the total number of valence electrons in beryllium hydride is 2 + (1 x 2) = 4 electrons.