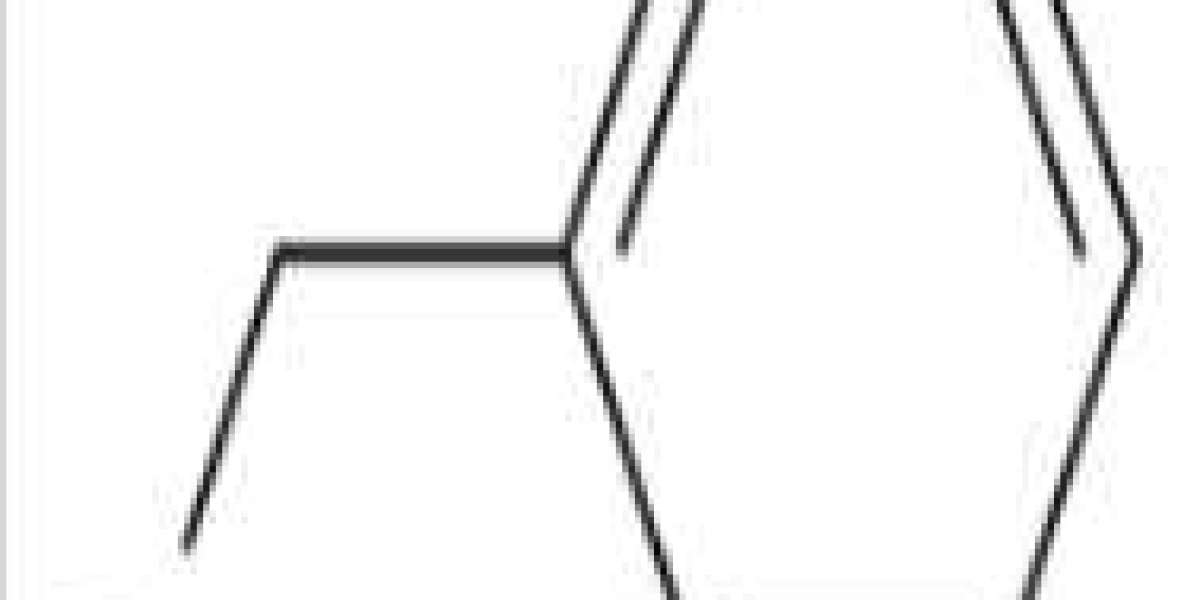
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
Benzyl bromide can be synthesized by the bromination of toluene under conditions suitable for a free radical halogenation:
Benzyl bromide is used in organic synthesis for the introduction of the benzyl groups when the less expensive benzyl chloride is insufficiently reactive.[6] [7] Benzylations are often achieved in the presence of catalytic amounts of sodium iodide, which generates the more reactive benzyl iodide in situ.[3] In some cases, benzyl serves as protecting group for alcohols and carboxylic acids.
Benzyl bromide is a strong lachrymator and is also intensely irritating to skin and mucous membranes. Because of these properties, it has been used in chemical warfare, both in combat and in training due to its irritating yet non-lethal nature.
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
Benzyl bromide is a member of the class of benzyl bromides that is toluene substituted on the alpha-carbon with bromine. It has a role as a lachrymator.
Benzyl bromide is an organobromide compound. It is used in organic synthesis for the introduction of the benzyl protecting group for alcohols and carboxylic acids. Benzyl bromide is a also strong lachrymator, as well as being intensely irritating to skin and mucous membranes. Because of these properties, it has been used as a war gas. Bromine is a halogen element with the symbol Br and atomic number 35. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock.
BENZYL BROMIDE reacts with water, alcohols, common metals (except nickel and lead), bases, amines and oxidizing agents. (NTP, 1992). This material stored over activated 4A molecular sieve burst a bottle due to condensation-polymerization reaction with generation of HBr gas
Benzyl bromide is a reagent and building block consisting of a benzene ring substituted with a bromomethyl group.
Benzyl Bromide is known as 100-39-0, Bromophenylmethane, 1-Bromotoluene, Cyclite, alpha-Bromotoluene, (Bromomethyl)benzene, Benzene, (bromomethyl) with Molecular Formula of C7H7Br and Molecular Weight of 171.03448. It is manufactured using reaction of benzyl alcohol and hydrobromic acid as well as action of bromine on toluene in UV light. It is available in form of clear, refractive liquid/colorless to yellow liquid and has sharp; pungent odor. Its properties include Boiling Point of 201°C, Melting Point of -3°C, Density/Specific Gravity of 1.7380 @ 25°C. Further, it has solubility in carbon tetrachloride, benzene and water (385 mg/liter @ 25°C).