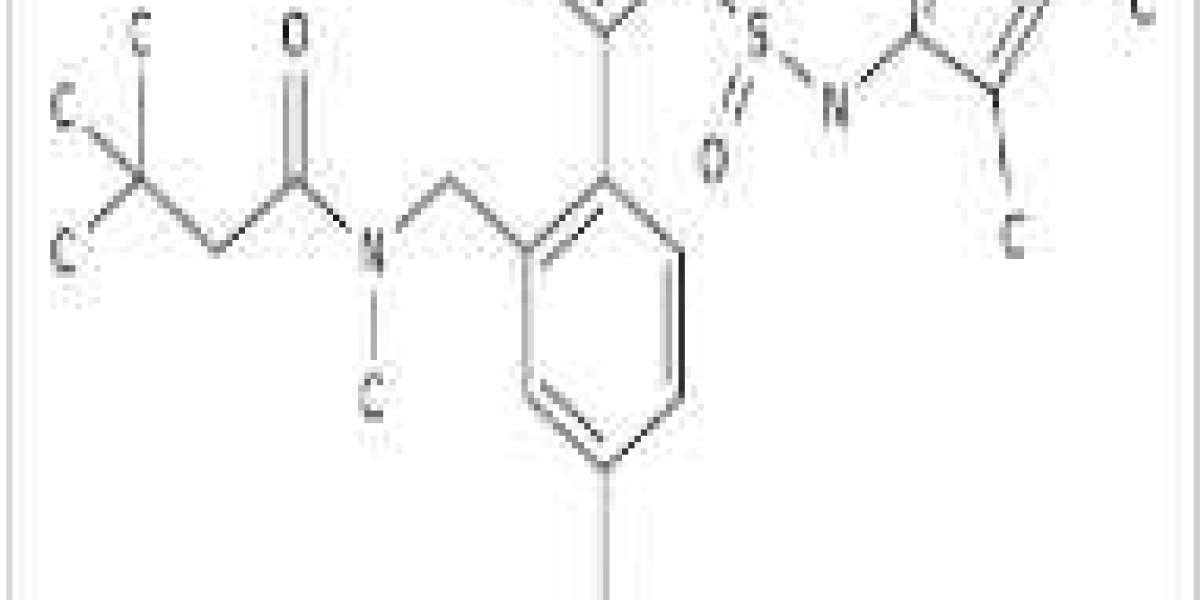
Edonentan is a selective antagonist of the endothelin receptor A [1-2]. It is a structural analogue of BMS-193884. ETA antagonists induce vasodilation and cardiac inhibition. Edonentan's pharmacodynamics supported oral administration.
Edonentan, also known as BMS-207940, is a very potent and selective ETA endothelin receptor antagonist (Ki= 10 pM) that shows 80000-fold selective for ETA vs ETB.
Edonentan monohydrate is a chemical compound that has gained significant attention in the scientific community due to its potential therapeutic and environmental applications. It is a small molecule inhibitor of the endothelin receptor, which plays a crucial role in regulating blood pressure and vascular tone.
A method of treating glaucoma in a subject in need thereof, comprising:
injecting a composition comprising a therapeutically effective amount of edonentan or a pharmaceutically acceptable salt thereof, into an optical tissue of said subject having glaucoma.
A method of treating glaucoma in a subject in need thereof, comprising:
injecting a composition comprising a therapeutically effective amount of edonentan or a pharmaceutically acceptable salt thereof, into an optical tissue of said subject having glaucoma.
The present disclosure relates to the field of medicine and the treatment of ocular disease. More specifically, the present disclosure relates to the use of Edonentan and A-182086 endothelin receptor antagonists in the treatment or amelioration of glaucoma, diabetic retinopathy (DR), retinal vein occlusion (RVO), non-arteritic anterior ischemic optic neuropathy (NAION), arteritic anterior ischemic optic neuropathy (AION), and retinopathy of prematurity (ROP).
Edonentan is a highly selective and very potent endothelin A receptor antagonist. Edonentan was developed as a second-generation analog following the discontinuation of the first clinical candidate, BMS-193884, which was being developed for the treatment of congestive heart failure (CHF). Edonentan was in phase I trials by April 2002, but its development was discontinued.
The method comprises contacting an optical tissue in a subject with a composition comprising a therapeutically effective amount of Edonentan or A-182086, or a pharmaceutically acceptable salt thereof, in which the endothelin receptor antagonist is Edonentan or A-182086. Such antagonist or its pharmaceutically acceptable salt can be in a crystalline form or an amorphous form, each of which can be for pharmacologically acceptable use.
In some embodiments, contacting comprises administering a topical composition to a surface of an eye or a portion thereof. In other embodiments, contacting comprises injecting Edonentan or A-182086 into an eye generally or in a specific area thereof.
In some embodiments, the endothelin receptor antagonist or a pharmaceutically acceptable salt thereof is Edonentan. In other embodiments, the endothelin receptor antagonist or a pharmaceutically acceptable salt thereof is A-182086. In either case, the antagonist or its pharmaceutically acceptable salt can be in a crystalline form or an amorphous form, each of which can be for pharmacologically acceptable use.