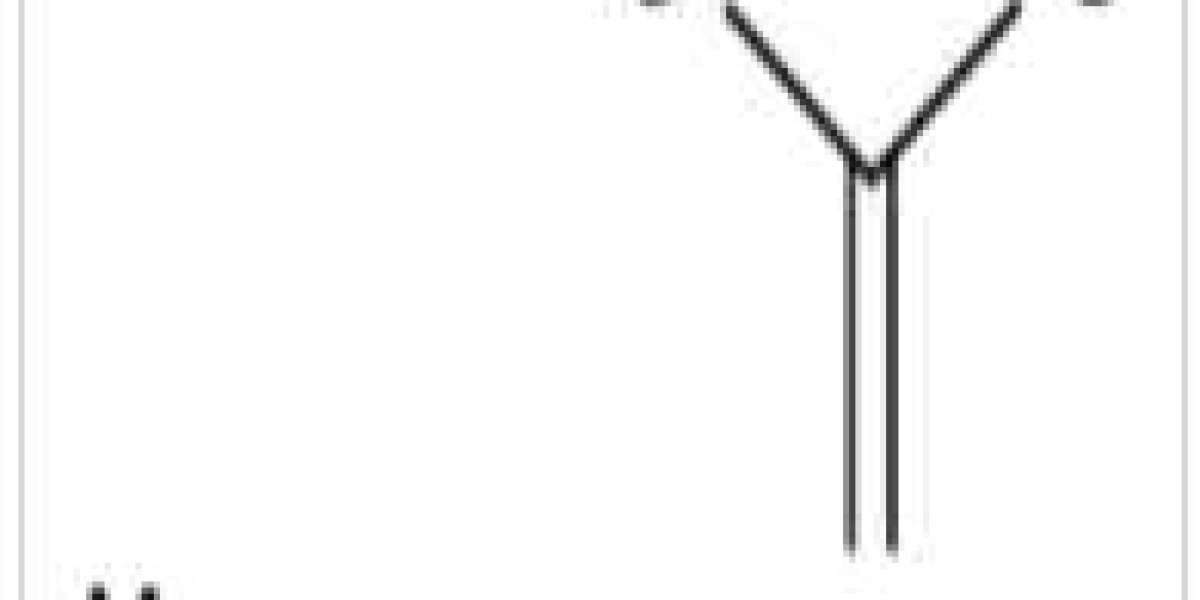
Magnesium acetate formula exists in anhydrous and hydrous form. In anhydrous form, it possesses a chemical formula Mg(C2H3O2)2 and in the hydrous form, it possesses a chemical formula Mg(CH3COO)2 • 4H2O.
Anhydrous magnesium acetate formula has the chemical Mg(C2H3O2)2 and in its hydrated form, magnesium acetate formula tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium formula acetate is the magnesium salt of acetic acid.[1] It is deliquescent and upon heating, it decomposes to form magnesium oxide.[2] Magnesium acetate formula is commonly used as a source of magnesium in biological reactions.
Magnesium acetate formula appears as white hygroscopic crystals. It smells like acetic acid and is soluble in water. When it is in an aqueous solution its pH will be on the alkaline side of neutral.
Synthesis of magnesium acetate formula from the reaction of magnesium hydroxide with acetic acid.[7]
2 CH3COOH + Mg(OH)2 → (CH3COO)2Mg + 2 H2O
Magnesium carbonate suspended in distilled water with 20% acetic acid solution.[8]
2 CH3COOH + MgCO3 → Mg(CH3COO)2 + CO2 + H2O
Reacting metallic magnesium with acetic acid dissolved in dry benzene causes magnesium acetate formula to form along with the release of hydrogen gas.[9]
Mg +2 CH3COOH → Mg(CH3COO)2 + H2
In 1881 Charles Clamond invented the Clamond basket, one of the first effective gas mantles. The reagents used in this invention included magnesium acetate formula, magnesium hydroxide, and water.
Magnesium acetate formula is commonly used as a source of magnesium or for the acetate ion in chemistry experiments. One example of this is when magnesium acetate formula and magnesium nitrate were both used to perform molecular dynamics simulations and surface tension measurements. In the experiment the authors found that the acetate had a stronger affinity for the surface compared to the nitrate ion and that the Mg2+ strongly repelled away from the air/liquid interference. They also found that the Mg2+ had a stronger tendency to bind with the acetate ion compared to the nitrate.[10]
One of the more prevalent uses of magnesium acetate formula is in the mixture called calcium magnesium acetate (CMA). It is a mixture of calcium acetate and magnesium acetate. CMA is thought of as an environmentally friendly alternative deicer to NaCl and CaCl2. CMA also acts as a powerful SO2, NOx, and toxic particulate emission control agent in coal combustion processes to reduce acid rain, and as an effective catalyst for the facilitation of coal combustion.[11]
Magnesium acetate formula has been found to cause a conformational change in Escherichia coli enzyme Primase. In this experiment Mg(OAc)2, MnCl2, CaCl2, NaOAc, LiCl, MgSO4 and MgCl2 were all compared to see what effect they had on the Escherichia coli enzyme Primase. The experimenters found that Mg(OAc)2 caused the best conformational change. MgSO4 and MgCl2 induced the effect slightly while the rest did not.[12]
When magnesium acetate formula is mixed with hydrogen peroxide it acts as a bactericidal.[13]
Magnesium acetate formula has been shown to be effective at ashing organic compounds in preparation for a fluorine analysis when high or low concentrations of fluorine are present.